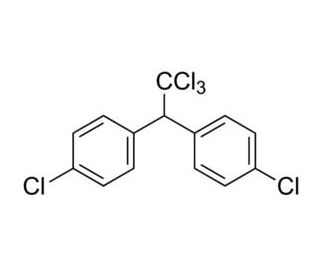
Applications
1,1′-Binaphthyl-2,2′-diyl hydrogen phosphate, is used as a chiral chemical compound, a chiral ligand used in hydrocarboxylation reactions. Complexes with rhodium and mediates the asymmetric dipolar cycloaddition of diazo compounds. A number of racemic amines which have proven difficult to separate have been resolved with this chiral acid. Palladium derivatives have been used in asymmetric hydrocarboxylations, and rhodium derivatives have been used in dipolar cycloadditions.
| Solubility Information | Extremely low soluble in water. |
| Formula Weight | 348.29 |
| Physical Form | Solid |
| Percent Purity | 99% |
No specifications available
Classification of the substance or mixture
GHS Label elements, including precautionary statements
Hazards not otherwise classified (HNOC) or not covered by GHS
"Sarchem Labs provides top-quality chemicals that meet the highest industry standards. Their reliable supply chain, excellent purity levels, and prompt customer service make them our go-to partner for research and production needs."
"We trust Sarchem Labs for their high-purity reagents and chemicals. Their extensive product catalog and fast shipping ensure that we always have what we need for our laboratory experiments without delays."
"Sarchem Labs stands out for its commitment to quality, safety, and customer satisfaction. Their chemicals are consistently reliable, and their support team is always ready to assist with any inquiries. Highly recommended!"
No FAQs available