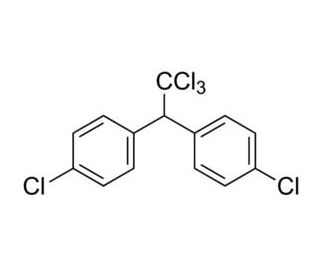
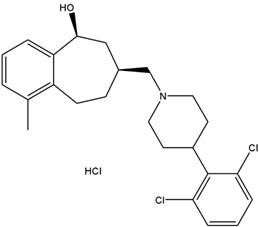
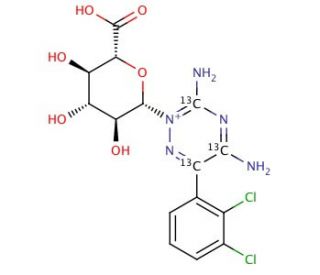
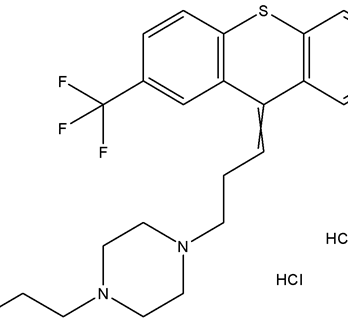
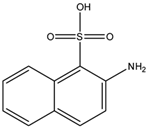
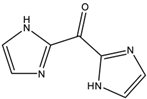
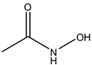
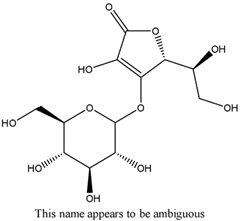
m-Xylene (PubChem CID: 7929) — also known as 1,3-dimethylbenzene, m-xylol, m-dimethylbenzene, meta-xylene, m-methyltoluene, 3-xylene, benzene 1,3-dimethyl, 1,3-dimethylbenzol, and Santosol 150 — is an aromatic hydrocarbon with multiple synonymous names.